
PharmaShots Weekly Snapshots (October 03–06, 2023)
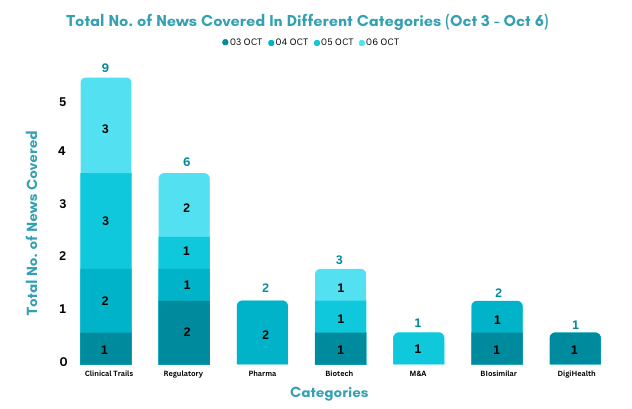
This week PharmaShots’ news was all about the updates on clinical trials, regulatory, biotech, pharma, M&A, biosimilar, and DigiHealth. Check out our full report below:

The US FDA has approved Novo Nordisk’ Rivfloza (nedosiran) for children aged ≥9 years and adults with primary hyperoxaluria type 1, based on the P-II trial (PHYOX 2) trial & interim data from P-III extension study (PHYOX 3)
Read more: Novo Nordisk
The US FDA has accepted the BLA and granted Priority Review for Rocket Pharmaceutical’s RP-L201 to treat Severe Leukocyte Adhesion Deficiency-I
Read more: Rocket Pharmaceutical
Agomab’ AGMB-129 Receives the US FDA’s Fast Track Designation for Fibrostenosing Crohn’s Disease & announced the initiation of the P-IIa trial (STENOVA) evaluating AGMB-129
Read more: Agomab
The NICE has recommended Boehringer Ingelheim’s Jardiance for symptomatic chronic heart failure with preserved or mildly reduced ejection fraction, based on the P-III (EMPEROR-Preserved) trial
Read more: Boehringer Ingelheim
The US FDA has granted Orphan Drug Designation to GC Biopharma's GC1126A for Thrombotic Thrombocytopenic Purpura
Read more: GC Biopharma

Syndax highlighted protocol-defined pooled analysis results from the (AUGMENT-101) trial results of Revumenib for r/r KMT2Ar Acute Leukemia which met its 1EPs & showed CR or a CR with CRh rate of 23% in 57 efficacy evaluable patients in the pooled KMT2Ar acute leukemia cohort
Read more: Syndax
Kashiv BioSciences enrolled the first patient in the P-III clinical study of ADL018 (biosimilar, omalizumab) for Chronic Spontaneous Urticaria
Read more: Kashiv BioSciences
Novartis highlighted pre-specified interim analysis results from the P-III study (APPLAUSE-IgAN) for Iptacopan to treat IgA nephropathy demonstrated the superiority of iptacopan vs PBO in proteinuria reduction
Read more: Novartis
PDS Biotech highlighted updated interim results from the P-II Trial (VERSATILE-002) results of PDS0101 + Keytruda for Head and Neck Cancer
Read more: PDS Biotech
BiomX completed patient dosing in part 2 of the P-Ib/IIa study for BX004 to treat Chronic Pulmonary Infections in patients with Cystic Fibrosis
Read more: BiomX
AstraZeneca highlighted P-III (T2NOW) trial results of Forxiga (dapagliflozin) for Type 2 Diabetes, which showed a significant reduction in A1C
Read more: AstraZeneca
The US FDA has placed a partial clinical hold on the new patient’s enrolment in the P-II trial (TELLOMAK) for Innate Pharma’ Lacutamab due to one fatal case of hemophagocytic lymphohistiocytosis
Read more: Innate Pharma
Neurocrine Biosciences highlighted P-III (CAHtalyst) pediatric study results of Crinecerfont in children and adolescents for Congenital Adrenal Hyperplasia showed a reduction from baseline in serum androstenedione from baseline
Read more: Neurocrine Biosciences
Regeneron highlighted two-year (PULSAR) trial results of Eylea HD (aflibercept) for wet age-related macular degeneration demonstrated sustained visual and anatomic improvements through 2yrs.
Read more: Regeneron
Tenaya Therapeutics dosed the first patient of TN-201 in the P-Ib trial (MyPeak-1) for MYBPC3-associated hypertrophic cardiomyopathy
Read more: Tenaya Therapeutics

The US FDA has cleared Anumana’s ECG-AI Algorithm to detect low ejection fraction in patients at risk of heart failure
Read more: Anumana

Sanofi & Janssen collaborated to develop and commercialize a vaccine candidate for extraintestinal pathogenic E. Coli
Read more: Sanofi & Janssen
ATUM extends its collaboration with Anagram Therapeutics to develop enzyme therapies for malabsorption and nutrient metabolism disorders
Read more: ATUM and Anagram Therapeutics
Twist Bioscience & Bayer collaborated to advance drug discovery where Bayer gets an exclusive right to license the Abs for commercialization in all global territories
Read more: Twist Bioscience & Bayer

Sanofi & Teva collaborated to co-develop and co-commercialize TEV ‘574 for inflammatory bowel disease
Read more: Sanofi & Teva
BridgeBio & Resilience collaborated to manufacture and advance BBP-812 for Canavan Disease and BP-631 for Congenital Adrenal Hyperplasia
Read more: BridgeBio & Resilience

Alvotech & Kashiv BioSciences collaborated to develop and commercialize AVT23, a proposed biosimilar to Xolair (omalizumab)
Read more: Alvotech & Kashiv BioSciences

Kyowa Kirin to acquire Orchard Therapeutics for ~$477.6M. The acquisitor enriches Kyowa Kirin’s portfolio and enables the development of numerous promising candidates with a clinically differentiated platform
Read more: Kyowa Kirin & Orchard Therapeutics
Related Post: PharmaShots Weekly Snapshots (September 25–29, 2023)
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














